Our Solutions
Where innovation meets
opportunity for patients in Europe.
We offer biopharmaceutical companies flexible strategic partnerships for the direct commercialization of their 1st pharmaceutical product in Europe.

Expand to Europe
Client Specific EU Hub
Central coordination of different platforms of services customized to our clients' needs, from development through to commercialization, and supported locally by a network of experienced and qualified partners.
Unlock France
Exploitant
Flexible Turnkey solutions to swiftly establish your locally compliant organization, surpassing conventional models ready-to-operate Exploitant asset with professional support and integration services.
Early Access Program
A unique, all-in-one solution for launching an early access program in one of Europe's most supportive regulatory environments.

Join the Expert Q&A Series
Critical Knowledge. Real Experts. Your Questions.
Get access to concise, high-impact PDFs where our industry veterans distill decades of experience to help you navigate Europe’s complex biotech and pharma landscape.
Each edition focuses on the critical knowledge you need—from regulatory hurdles to post-approval strategy—so you avoid costly missteps and move
forward with clarity.
You’re not just reading—you’re part of the dialogue. Each edition is shaped by questions from our community, and you can submit your own for future
Q&As.
Related articles
Related articles

5 Critical Considerations for French Market Entry
How a Pharmaceutical Company Cut 18 Months off the Standard Timeline for Establishing Its Authorized Local Subsidiary.
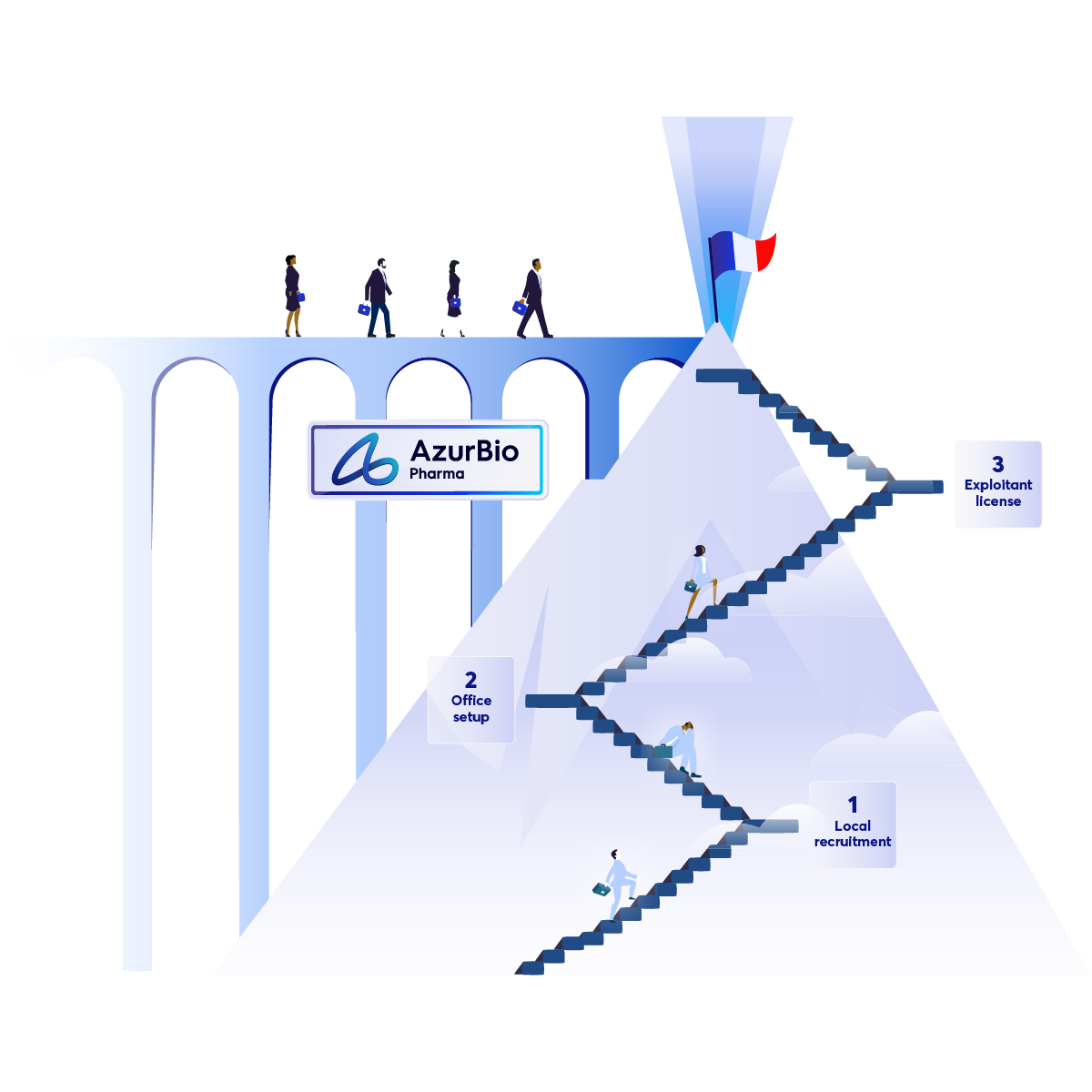
Make France your Next Success Story
Why Setting Up in France Feels Like Climbing a Mountain—and How AzurBio Pharma Can Help!

5 Critical Considerations before your first EMA interaction
A practical guide for biopharma companies preparing for their first EMA interaction. Learn 5 critical steps to reduce delays and align with EU regulat...

