
White Papers
5 Critical Considerations before your first EMA interaction
Get all the business experience needed for a successful first EMA interaction.
5 Critical Considerations before your first EMA interaction

Planning Your First EMA Interaction?
Start Here.
The European Medicines Agency (EMA) isn’t just the FDA with different forms. From pediatric plans to country-specific pricing, the EU brings its own set of rules—and risks.
This guide walks you through:
-
When and how to engage the EMA through Scientific Advice
-
Timing and structure of your Pediatric Investigation Plan (PIP)
-
Meeting CMC expectations well ahead of submission
-
Building a market-access strategy tailored to EU payers
-
Mapping key stakeholders—from KOLs to HTA bodies
Register to download
our guide
Discover our latest news
Articles
Launching of AzurBio Consulting
AzurBio Pharma Strengthens Its Foothold in European Regulatory Services with the Acquisition of A-pharmaconsult, Launching AzurBio Consulting.

Expert Q&As
First interactions with EMA - Q&A 1
What are the different regulatory pathways available when expanding to Europe and which one is the best fit for me?
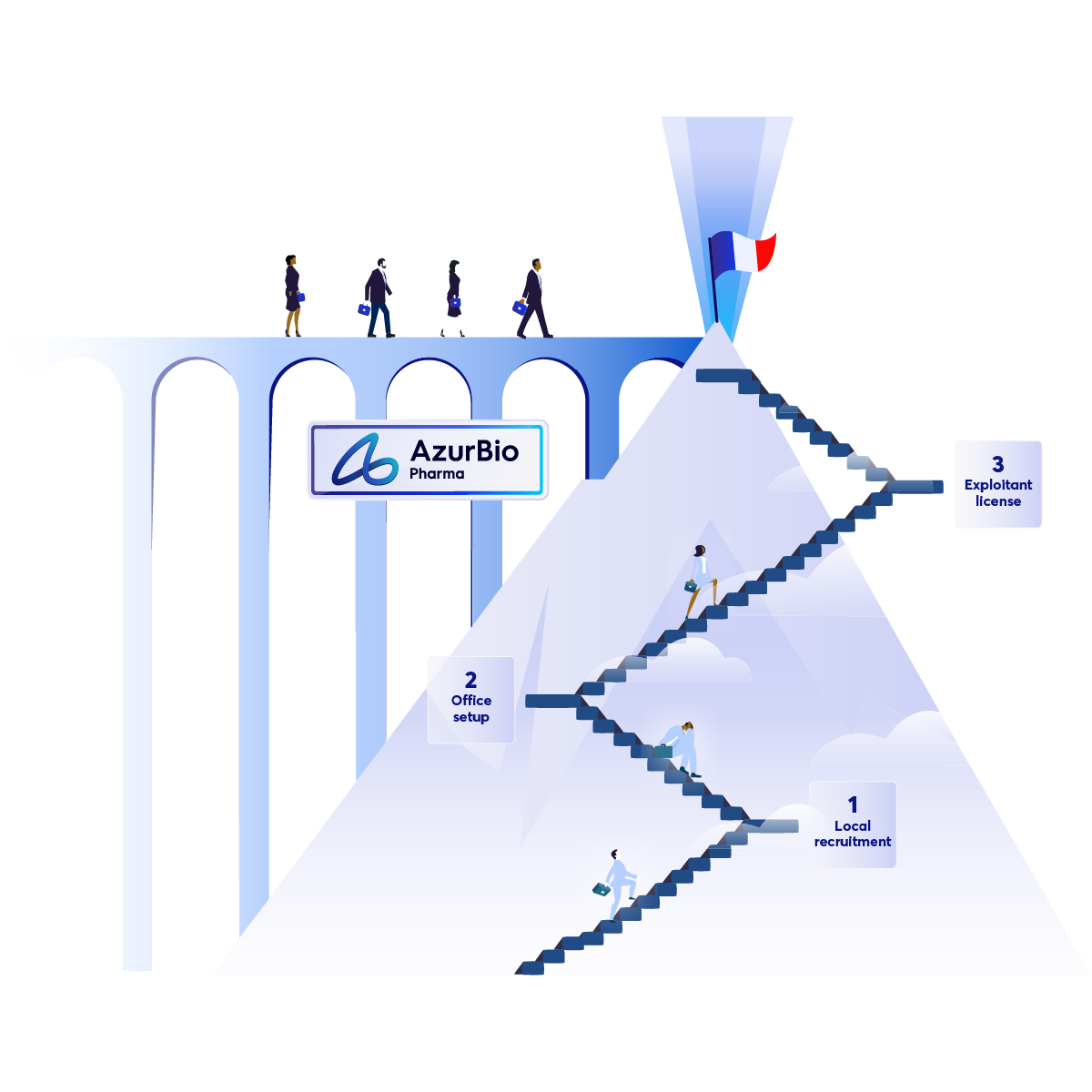
Articles
Make France your Next Success Story
Why Setting Up in France Feels Like Climbing a Mountain—and How AzurBio Pharma Can Help!
