Unlock France
Meeting France’s Exploitant requirements efficiently & compliantly.
Exploitant responsibility in France:
Choose from our 3 flexible models.

Build
Build Your Own Exploitant.
AzurBio to provide End-to-End Setup services.

Buy
Acquire Pre-Built Exploitant entity to become yours.
Acquire Ready-to-Operate Structure from us.

Borrow
Outsource the Exploitant
Role to us.
AzurBio to act as Your Exploitant.

Build
Confidently Navigate Your Path to Establish your Own in-House Exploitant.
End-to-end support for companies looking to create their own in-house Exploitant.
From regulatory setup to QMS implementation and inspection readiness

Your Goal
Obtain & secure Exploitant status for your French affiliate while minimizing internal resource demands and local recruitment needs, and maximizing regulatory success—ensuring a compliant, efficient, and inspection-ready setup from day one.Our Solution
- A specialized team with deep expertise in French regulatory requirements to lead your setup from concept to ANSM authorization and keep you on track.
- A tailored roadmap for building your Exploitant, aligned with your product strategy and commercialization goals.
- End-to-end support to obtain the ANSM Exploitant license as well as SOPs development, QMS design and implementation, and appointment of a Pharmacien Responsable.
- Full inspection readiness through mock audits, gap assessments, and compliance coaching.
- Seamless coordination with your internal teams and third-party partners to integrate the Exploitant structure into your wider EU operations.

Buy
Acquire a Ready-to-Operate Exploitant Structure.
A turnkey solution to accelerate setup and immediate alignment with French regulatory requirements.
Acquire a fully compliant, ready-to-operate Exploitant entity.

Your Goal
Fast-track your market entry in France by acquiring a fully compliant, ready-to-operate Exploitant structure—enabling immediate regulatory alignment without the delays of building from scratch.Our Solution
- A fully compliant ANSM-authorized Exploitant entity, ready for immediate deployment.
- Streamlined acquisition process with due diligence support to ensure a smooth transition and operational readiness.
- Existing QMS, SOPs, and infrastructure in place—minimizing setup time and regulatory risk.
- Optional provision of a Pharmacien Responsable and core operational team to support your launch.
- Ideal for companies seeking long-term presence in France without the delays of building an Exploitant from scratch or looking to derisk market entry in France.

Borrow
Use AzurBio as Your Exploitant Partner.
For a faster, low-risk market entry. We manage all regulatory responsibilities, allowing you
to focus on your product launch.

Your Goal
Accelerate your product launch in France by leveraging AzurBio as your Exploitant partner—eliminating or deferring the need to build your local Exploitant infrastructure while ensuring full regulatory compliance and operational readiness from day one.Our Solution
- A compliant, ANSM-authorized Exploitant structure, ready to support your product from day one.
- Immediate access to a regulatory, quality, and pharmacovigilance infrastructure—without the need to set up a local affiliate.
- Full regulatory representation before ANSM, including oversight of product lifecycle, promotional compliance, and batch monitoring.
- A complete QMS managed by AZB’s Pharmacien Responsable and expert team, aligned with French and EU standards.
- Seamless collaboration with your internal teams and partners to maintain timelines and regulatory continuity throughout commercialization
Understanding the Role of the Exploitant
This legally designated entity is responsible on the French market for:
- Ensuring Regulatory Compliance
- Supervising Batch tracking and recalls
- Overseeing Medical information
- Overseeing Pharmacovigilance
- Managing Advertising/promotional material compliance
incl. Charter on information provided for the promotion of medicinal products through prospecting or canvassing
The Exploitant’s responsibilities are supported by a dedicated organization and Quality Management System (QMS), which must ensure ongoing compliance and inspection readiness.
The designated site is regularly inspected by the ANSM, and the QMS must cover all processes related to the Exploitant’s operations and regulatory obligations.
The Exploitant is required to appoint a Pharmacien Responsable (Chief Pharmaceutical Officer), who holds personal and legal accountability for the company's pharmaceutical activities and compliance with French pharmaceutical regulations..
To obtain Exploitant status, a company must submit a comprehensive dossier to the ANSM, detailing the organizational structure, quality systems, and procedures in place to manage the responsibilities outlined above. This includes information about the appointed Pharmacien Responsable, facilities, and any subcontracted activities. The process requires meticulous preparation and a deep understanding of French regulatory expectations.
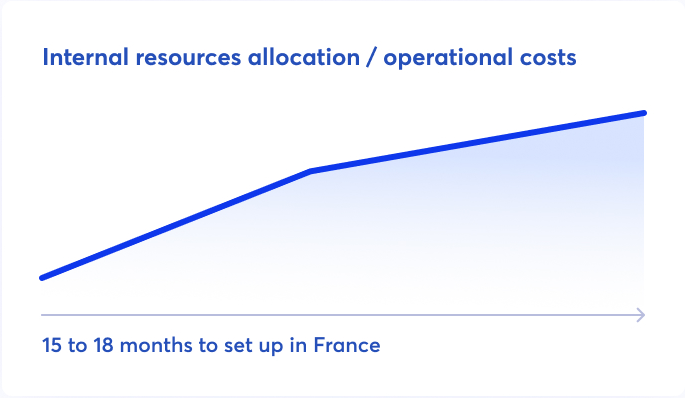
Establishing a commercial subsidiary in France is a time-consuming and resource-intensive process that can take up to 18 months.
It demands substantial local expertise, internal resource allocation, and local recruitment, even for part-time roles, to set-up and organize an ANSM-authorized Exploitant structure.
Companies with little or no initial presence in France are seeking customized solutions to rapidly establish their own Exploitant subsidiary for a swift business start, particularly to accelerate launch of their first product through an early access program.
Join the Expert Q&A Series
Critical Knowledge. Real Experts. Your Questions.
Access concise, high-impact PDFs where our experts break down the essentials of navigating France’s complex regulatory and market landscape.
Each edition focuses on key themes for biotech companies entering or growing in France—from setting up operations to staying compliant and inspection-ready—so you can move forward with clarity and confidence.
This is an interactive series—your questions shape the content, ensuring every edition addresses the real-world challenges of expanding into Europe.
Related articles
Related articles

5 Critical Considerations for French Market Entry
How a Pharmaceutical Company Cut 18 Months off the Standard Timeline for Establishing Its Authorized Local Subsidiary.

Early Access Programs in France
How a US Biotech Accelerates the Availability of Its Rare Disease Treatment and Optimizes the Timing for Establishing a Long-Term Market Presence.
Make France your Next Success Story
Why Setting Up in France Feels Like Climbing a Mountain—and How AzurBio Pharma Can Help!

